
Effect Of Water Composition On Coffee Extract – When making coffee, people often only pay attention to factors such as coffee powder, pressure, and temperature … but forget the critical role of water in coffee extraction.
Water makes up 98-99% of a cup of coffee; the type of water and the ingredients in the water are the determining factors for the taste of the cup. Therefore, to create a cup of coffee with a delicious taste, it is essential to understand the components and properties of water.
Water with no minerals other than calcium or magnesium carbonate (CaCO3, MgCO3) contains equal amounts of hardness and alkalinity (in hardness equivalent units). A total hardness that is much higher than alkalinity indicates that there is a significant amount of sulfate present in the water. Let’s learn about the Effect Of Water Composition On Coffee Extract with Helena Coffee Vietnam.
Extraction impact of water composition
Hardness and alkalinity are identical in water hardness, including no minerals other than calcium or magnesium carbonate (CaCO3, MgCO3) (in hardness equivalent units). A total hardness substantially higher than alkalinity implies that the water contains a significant amount of sulfate.
After the roasted and ground coffee beans, water and its minerals are possibly the most significant factors in the quality of a coffee beverage (Navarini and Rivetti, 2010).
Water is routinely treated to lessen its hardness to avoid mineral precipitation due to its potential for scaling.
Another aspect that has an equal impact on extraction as water composition is the mass ratio of water to roasted and ground coffee beans, which can vary by up to a factor of ten depending on the extraction process.
An espresso, for example, can be made using a dry coffee mass-to-beverage mass ratio of 1:2
Although the impact of water composition on filter coffee has been studied since 1950, espresso has just been studied for the last 20 years. Three different situations are discussed in the papers below:
- At high alkalinity (>100 ppm), more of the acids extracted from coffee are neutralized by hydrogen carbonate, resulting in carbonic acid, which, depending on pressure and temperature, can decrease as carbon dioxide. Carbon dioxide production during extraction adds to the resistance and extends the extraction time under otherwise identical conditions (Fond, 1995; Rivetti et al., 2001). A practical example of espresso extraction with water treated by an ion exchanger that affects total hardness or alkalinity may be found in Section 6.2.
- When water is heated, high alkalinity and low hardness cause an elevation in pH; this effect is amplified when sodium is present. It has been claimed that decreased solubility of carbohydrates in water at high pH is the reason for more excellent resistance during extraction (Navarini and Rivetti, 2010)
- No increase in percolation time was seen for high hardness and equal (or lower) alkalinity (Fond, 1995; Rivetti et al., 2001).
Acidity Buffering in Coffee
The perception of acidity in coffee has been extensively researched (for an overview, see Gloess et al., 2013).
The association of sensory perception of acidity with titrable acidity of coffee extracts is the most widely mentioned phenomenon (titration to a pH of 6.6, which corresponds to mouth pH).
This would also make sense from a chemical standpoint, as noted by Clifford (1988): “In effect, the acid’s response with the receptor is a titration, and hence quite similar to the procedure used in determining titratable acidity.”
Because water’s alkalinity combines with coffee’s extracted acids, it effectively neutralizes some acids.
Water extracts can account for as much as 30 percent or 11 percent of the titrable acidity detected in the filter or lungo espresso extractions, according to data fromGloessetal. (2013) (authors’ measurements and estimates).
These percentages were calculated using water with a 50 ppm CaCO3 (1 mmol/L) alkalinity, which was utilized to extract a beverage from a medium to dark roasted coffee (Guatemala, Antigua, la Ceiba, 80 Pt on Colorette 3b, w45 Pt on Agtron M-Basic scale).
As a result of Clifford’s idea, a simple equation for perceived acidity may be developed, in which the perceived edge of coffee tea quals acidity extracted from quality coffee beverages is reduced by the alkalinity of the water.
Water with the Optimal Composition for Coffee Extraction
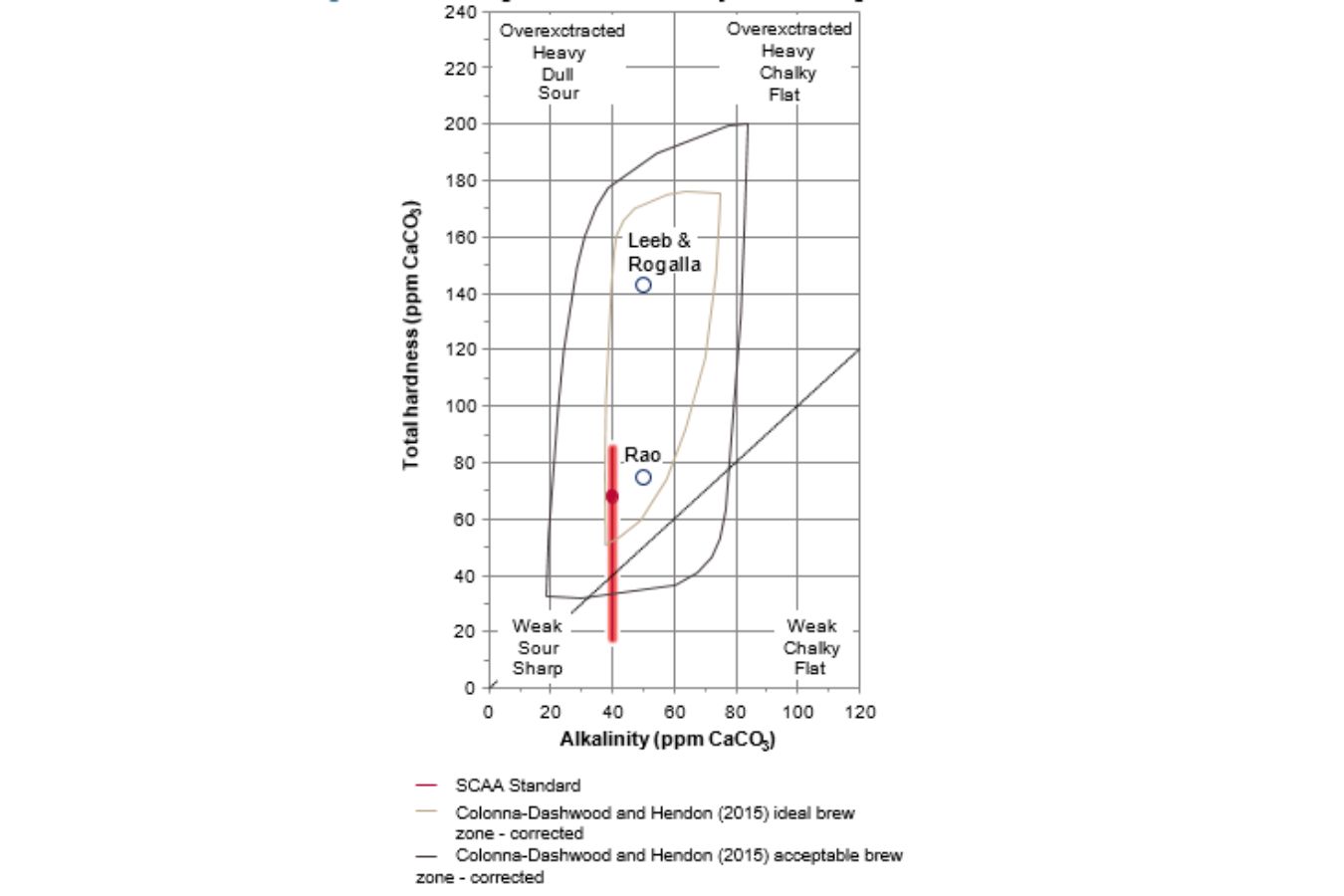
The “SCAA Standard: Water for Brewing Specialty Coffee” is the coffee industry’s most widely used sensory property standard (2009). It specifies a total hardness of 68 ppm CaCO3 (with an acceptable range of 17e85 ppm CaCO3), alkalinity of 40 ppm CaCO3, and a pH of 7 as the optimal extraction conditions (good range of pH 6.5e7.5).
The optimum is marked with a circle in Fig. 16.3, denoted by a red line (pH is not considered in the graph). It also specifies a sodium goal value of 10 mg/L, even though sodium is only detectable at concentrations greater than 250 mg/L. (Pohling, 2015).
The phrase “total chlorine,” which should be zero, only relates to chlorine gas, hypochlorite (OCl), and disinfectants that taste lousy. On the other hand, tasteless ion chloride (Cl) is not restricted.
The optimal total dissolved solids (TDS) value is reported to be 150 mg/L; however, the typical method of calculating TDS using a conductivity meter has a 30 percent inaccuracy.
Treatment of water
In this section, we’ll show you how to describe water treatment processes in terms of hardness and alkalinity in a new and practical approach. Water treatment technologies can be divided into five groups in general: l Filtration: Particles, bacteria, and organic substances that cause off-flavors to be removed.
Cation exchange [e.g., magnesium and calcium ions are exchanged for hydrogen ions (protons) or sodium ions] combined cation. Anion exchangers produce deionized, nearly pure H2O mixed with tap water to modify its mineral content.
l Reverse osmosis: Nonselective removal of all dissolved particles by filtration through a semipermeable membrane, which is permeable to water but not to other components in water.
Concerning coffee extraction, odor-free, clean drinking water can be accurately characterized by its hardness (as a measure of extraction efficiency) and alkalinity (as a measure of its acid buffering capacity).
Fig. 16.4 provides an overview of the changes in hardness and alkalinity resultant from the most common treatment methods, using two different initial high-quality water compositions, 1 and 2 :
a: SoftenerdCation exchanger: Ca2þ and Mg2þ for potassium (Kþ) or sodium (Naþ) only affects hardness and is, therefore, vertically oriented in Fig. 16.4. The alkalinity is not affected.
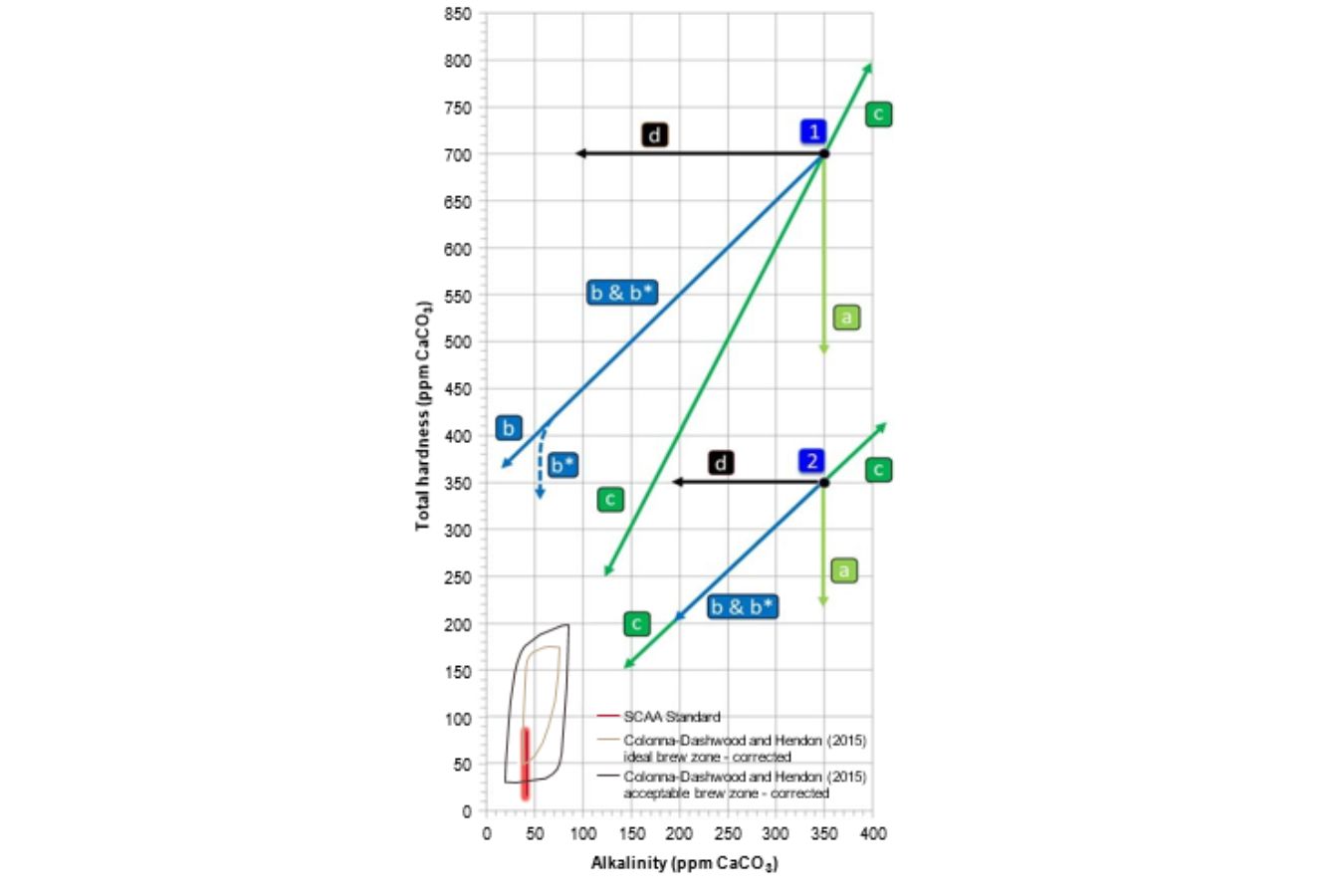
b: DecarbonizereCation exchanger: Ca2þ and Mg2þ for Hþdoriented diagonally with a slope of 1. The net effect is that the change in alkalinity equals the change in hardness. See Section 6.2 for a discussion of the effect of this treatment on espresso extraction.
b*: Combination of decarbonizer ( b -type) with a small fraction of softener ( a -type).
c: Demineralizer: Reverse osmosis (RO) or deionizer by ion-exchanged removing ions irrespective of the initial composition; RO can also increase the mineral content by mixing concentrated water with untreated water before the membrane oriented toward the point of origin (0/0) or away from it.
d: Dealkalizer: Anion exchanger: HCO3 for Cl (not yet commercially available for coffee applications) or addition of a strong acid (e.g., HCl). This will lead to a reduction of the alkalinity without affecting its total hardness.
e: not shown: Cation exchange of Ca2þ for Mg2þddoes does not change either hardness or alkalinity, so both values remain constant. However, the mineral composition of the water will be altered.


